MC4R - a key regulator of body weight
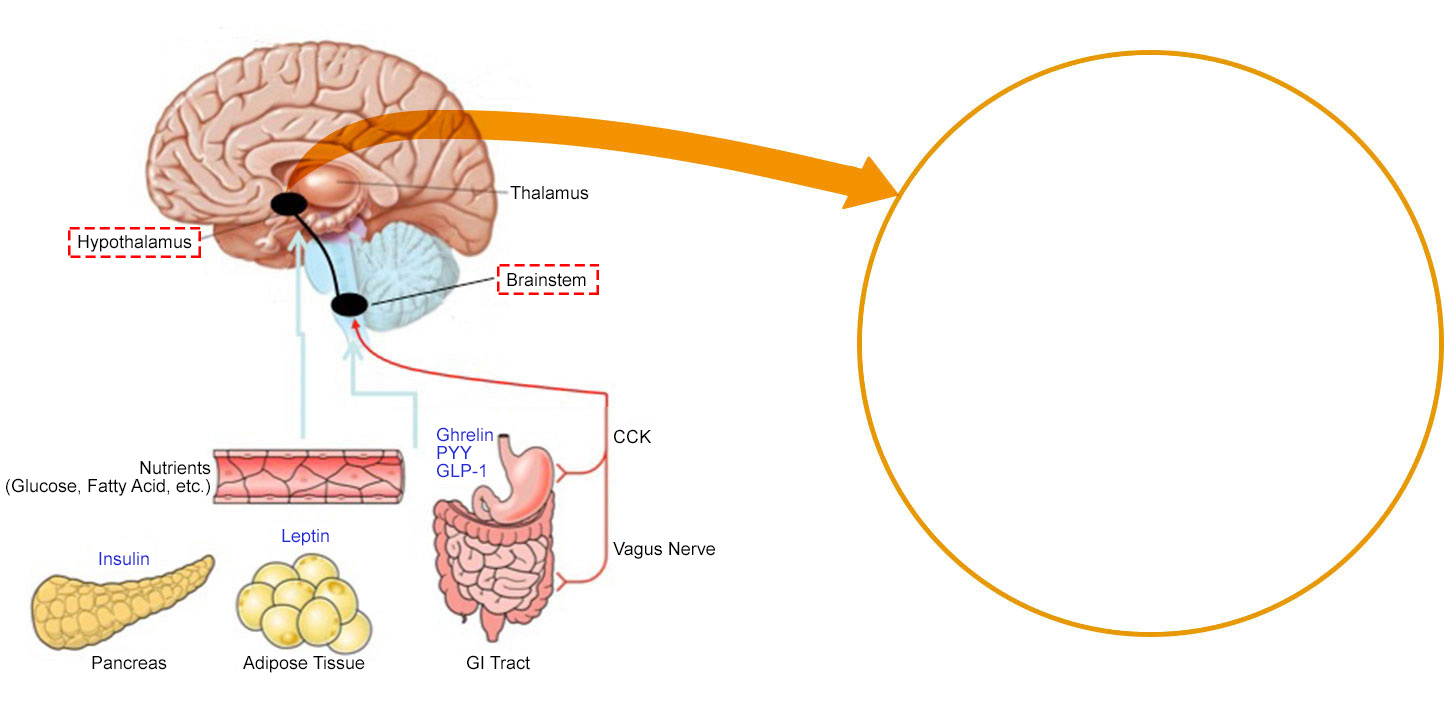
The hypothalamus is a key part of the brain that integrates neural and hormonal signals from peripheral organs such as the gut and adipose tissue to regulate body weight. Critically, the hormone leptin, which is produced by adipose tissue, is an indicator of nutritional state. Fasting leads to a fall in leptin which triggers a set of responses in the brain which act to restore energy balance.

Leptin acts on neurons within the hypothalamic arcuate nucleus which act as primary sensors of alterations in energy stores. In the fasting state, a fall in leptin stimulates neurons expressing agouti related peptide (AgRP) to increase food intake. At the same time, leptin inhibits POMC neurons, reducing the amount of α-MSH (melanocyte stimulating hormone), which normally suppresses food intake. These signals are integrated by second order neurons which express the melanocortin-4 receptors (MC4R).
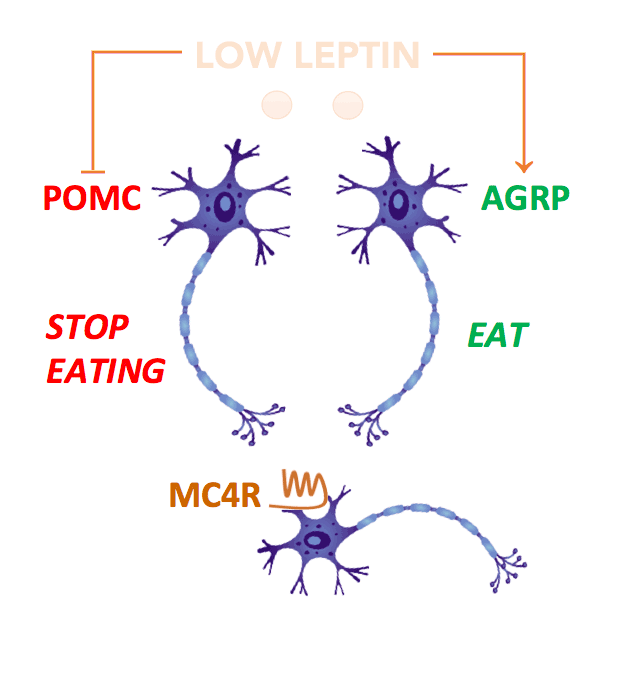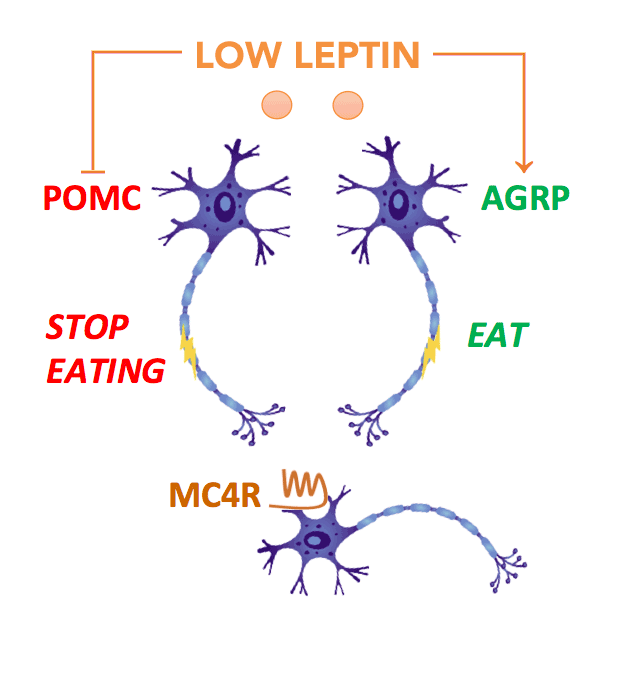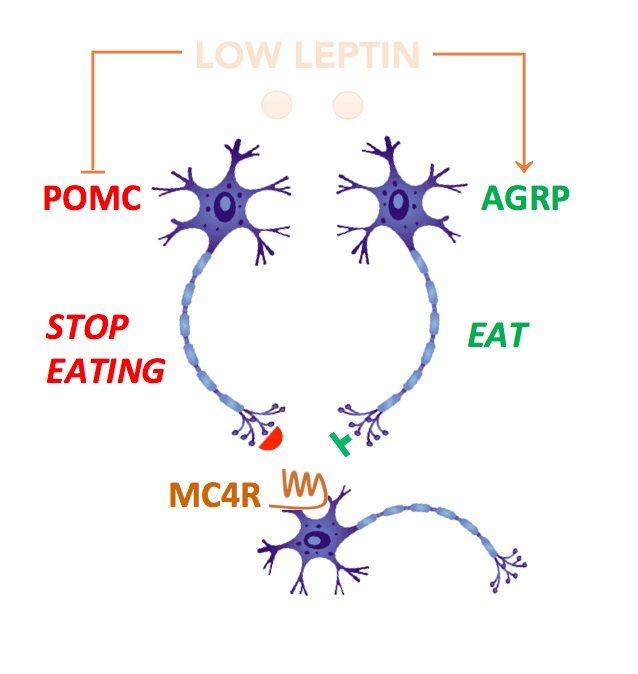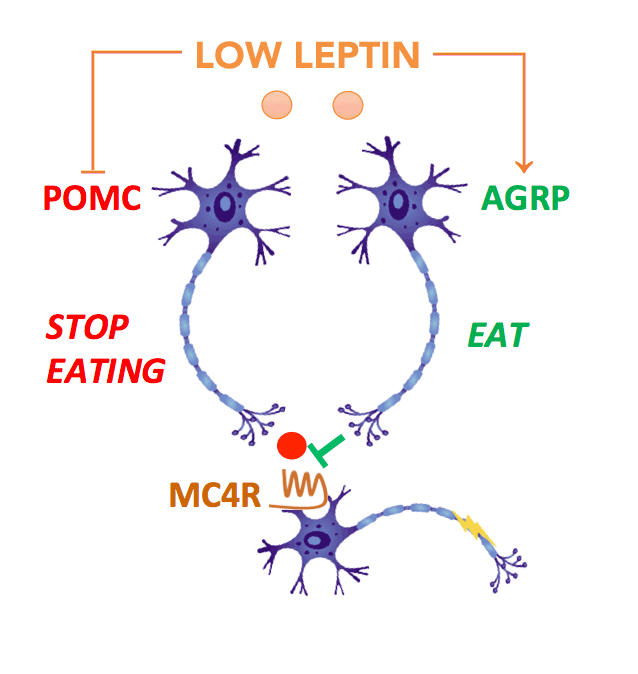

In this way, fasting leads to an increase in food intake which restores energy balance. Disruption of MC4R in mice leads to obesity demonstrating that this pathway is critical for the control of appetite and body weight. These findings in animals paved the way for studies in humans and in 1998, we and colleagues in France identified the first mutations in the MC4R gene in people with severe obesity from early childhood.
Genetics
Heterozygous mutations in MC4R are associated with dominantly inherited obesity and have been reported in obese people from various ethnic groups. The prevalence of MC4R deficiency is approximately 2-5% in severely obese children, 1% in severely obese adults and approximately 1 in 500 in the population making MC4R deficiency the commonest monogenic cause of obesity. Homozygous mutations in MC4R and double heterozygous mutations (different mutations on both alleles) are rare but cause very severe obesity. In clinical cohorts, approximately 25% of MC4R mutations are heterozygous frameshift or nonsense mutations which cause a complete loss of function (LOF). The remainder are missense mutations, most of which reduce the expression of MC4R on the cell membrane and/or its ability to signal by generating cyclic AMP (cAMP). However, approximately 20% of MC4R missense mutations were considered non-pathogenic, because they do not affect the main signalling pathway (Gαs-mediated cAMP production). In recent work, using a comprehensive set of assays, we have shown that these mutations (often called wild-type (WT)-like in some papers), all impair the function of MC4R by affecting a range of cellular mechanisms. They can impair receptor trafficking and internalisation, decrease β-arrestin recruitment and/or MC4R homodimerization. This work establishes that these mechanisms can regulate MC4R function to a level that is physiologically relevant. An up-to-date functional classification of all reported MC4R variants is shown below (updated Feb 2025).
MC4R Deficiency
People with genetic mutations in MC4R (often termed MC4R deficiency) tend to gain weight from early childhood. As well as increased fat mass, MC4R deficient people also have an increase in lean mass and increased linear growth in childhood. As such, people can appear “big-boned”. The increased growth may in part be mediated by disproportionate early hyperinsulinaemia. The main clinical feature in MC4R deficiency is hyperphagia, an increased drive to eat as well as impaired satiety (less fullness after a meal). We have shown that the severity of receptor dysfunction seen in cells is associated with the severity of hyperphagia. The severity of many of the clinical features may reduce with time. Thus, adults report less intense feelings of hunger and are less hyperinsulinemic than children with the same mutation.



Adults with MC4R deficiency have a lower prevalence of hypertension and significantly lower blood pressure than people of the same age and weight. The finding of lower 24-hour urinary noradrenaline excretion, lower heart rate and lower measures of sympathovagal tone suggest that impaired sympathetic nervous system activation is the main explanation for the lower blood pressures seen in MC4R deficiency. Weight loss is hard to achieve in MC4R deficiency as people are often less responsive to diet and exercise. One reason may be that the normal physiological response to caloric restriction requires an intact leptin-melanocortin system. Bariatric surgery, specifically Roux-en-Y-bypass surgery can be effective in people with heterozygous MC4R mutations but may not be effective in people with homozygous mutations. Semaglutide and Tirzepatide (GLP-1 receptor agonists) can lead to weight loss in patients with MC4R deficiency.
Publications
- Zorn S, Bounds R, Williamson A, Lawler K, Hanseen R, Keogh J, Henning E, Smith M, Fielding BA, Umpleby AM, Yasmeen S, Marti-Solano M, Langenberg C, Wabitsch M, Collet T-H, Farooqi IS. Obesity due to MC4R deficiency is associated with reduced cholesterol, triglycerides and cardiovascular disease risk, Nature Medicine. 2025 Oct. Online ahead of print.
- Bhatnagar P, Ahmad NN, Li X, . Tirzepatide leads to weight reduction in people with obesity due to MC4R deficiency,Nature Medicine. 2025 Aug. Online ahead of print.
- Wade KH, Lam BYH, Melvin A, Pan W, Corbin LJ, Hughes DA, Rainbow K, Chen JH, Duckett K, Liu X, Mokrosiński J, Mörseburg A, Neaves S, Williamson A, Zhang C, Farooqi IS, Yeo GSH, Timpson NJ, O’Rahilly S. Loss-of-function mutations in the melanocortin 4 receptor in a UK birth cohort, Nature Medicine. 2021 Jun;27(6):1088-109
- Clément K, van den Akker E, Argente J, Bahm A, Chung WK, Connors H, De Waele K, Farooqi IS, Gonneau-Lejeune J, Gordon G, Kohlsdorf K, Poitou C, Puder L, Swain J, Stewart M, Yuan G, Wabitsch M, Kühnen P. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials, Lancet Diabetes Endocrinol. 2020;8(12):960-970.
- Brouwers B, Mendes de Oliveira E, Marti-Solano M, Monteiro FBF, Laurin SA, Keogh JM, Henning E, Bounds R, Daly CA, Houston S, Ayinampudi V, Wasiluk N, Clarke D, Plouffe B, Bouvier M, Babu MM, Farooqi IS, Mokrosiński J. Human MC4R variants affect endocytosis, traffickingand dimerization revealing multiple cellularmechanisms involved in weight regulation, Cell Reports, 23 March 2021, 34, 108862
- Lotta LA, Mokrosinski J, Mendes de Oliveira E, Li C, Sharp SJ, Luan J, Brouwers B, Ayinampudi V, Bowker N, Kerrison N, Kaimakis V, Hoult D, Stewart ID, Wheeler E, Day FR, Perry JRB, Langenberg C, Wareham NJ, Farooqi IS. Human Gain-of-Function MC4R Variants Show Signaling Bias and Protect against Obesity, Cell, 2019 April;177(3):597-607.e9.
- Iepsen EW, ZhangJY, Thomsen HS, Hansen EL, Hollensted M, Madsbad S, Hansen T, Holst JJ, HolmJ-C, Torekov SS. Patients with Obesity Caused by Melanocortin-4 Receptor Mutations Can Be Treated with a Glucagon-like Peptide-1 Receptor Agonist, Cell Metabolism, 2018 July; 28(1): 23-32.e3.
- Collet T-H, Dubern B, Mokrosinski J, Connors H, Keogh JM, Mendes de Oliveira E, Henning E, Poitou-Bernert C, Oppert J-M, Tounian P, Marchelli F, Allili R, Le Bihan J, Pépin D, Lacorte J-M, Gottesdiener A, Bounds R, Sharma S, Folster C, Henderson B, O’Rahilly S, Stoner E, Gottesdiener K, Clément K, Farooqi IS, van der Ploeg L. Evaluation of a Melanocortin-4 Receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency, Molecular Metabolism, July 2017;6(10):1321-1329.
- Hatoum I, Stylopoulos N, Vanhoose A, Boyd K, Yin D, Ellacott K, Ma L, Blaszczyk K, Keogh J, Cone R, Farooqi IS, Kaplan L. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J Clin Endocrinol Metab. 2012 Jun;97(6):E1023-31.
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O’Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med.2009;360(1):44-52. Stutzmann F, Tan K, Vatin V, Dina C, Jouret B, Tichet J, Balkau B, Potoczna N, Horber F, O’Rahilly S, Farooqi IS, Froguel P, Meyre D. Prevalence of MC4R deficiency in Europeans and their age-dependant penetrance in multi-generational pedigrees. Diabetes 2008;57:2511-8.
- Farooqi IS, Keogh J, Yeo G, Lank E, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 2003;348:1085-95
- Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:113-4. Yeo GS*, Farooqi IS*(*joint first authors), Aminian S, Halsall DJ, Stanhope RG and O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet 1998;20:111-112.